Anderson–Fabry disease (AFD) is a rare X-linked disorder caused by defects of the alpha-galactosidase (α-Gal) enzyme. Mutations in the Alpha Galactosidase gene (GLA), which encodes for α-Gal, affect synthesis, trafficking, folding, degradation and enzymatic activity of α-Gal resulting in progressive intracellular accumulation of globotriaosylceramide (Gb3). Intracellular Gb3 and related glycosphingolipids accumulation leads to organ/tissue damage potentially affecting cardiovascular, renal, gastrointestinal, cerebrovascular, neurologic, auditory, ocular and cutaneous systems. Recent evidence supports the hypothesis of a tissue-specific, mutation-dependent ‘affinity’ for Gb3 storage. The AFD clinical phenotype is characterized by variability in the age of onset and severity and can be severe and early in classic forms of AFD or mild and later in variant forms. Replicated evidence demonstrates that carriers of certain mutations in the GLA gene develop preferential, albeit nonexclusive, cardiac, renal and neurologic phenotypes: for example p.(Asn215Ser) and p.(Phe113Ile) are invariably associated with late-onset hypertrophic cardiomyopathy-like (HCM-like) phenotype.
 |
| Fabry-Anderson disease |
Hemizygous males with the classic form of AFD demonstrate low or absent enzyme activity. The patients typically develop signs and symptoms in childhood or adolescence (delayed puberty and growth, gastrointestinal symptoms, corneal opacities, angiokeratomas, acroparesthesias/neuropathic pain).
Thickening of the left ventricular (LV) wall, renal failure, vascular complications, cryptogenic stroke and transient ischemic attack (TIA) are features that become noticeable only during adulthood. Heterozygous females with the classic form of AFD may experience later onset of the disease and typically exhibit heterogeneous and milder phenotypes. They may have normal residual enzyme activity limiting the role of testing α-gal actosidase activity as diagnostic assay. The nonclassic AFD patients have symptoms mostly limited to single organs and their phenotypes are termed cardiac, renal and neurological variants. The nonclassical variants of the disease lead to difficult clinical dilemmas increasing the risk of misdiagnosis.
Major complications of AFD are renal failure, HCM-like cardiac involvement and cryptogenic stroke. Data on the long-term evolution of the disease in untreated patients provide limited information: a few studies performed before introduction of enzyme replacement therapy (ERT) in 2001 had shown that men have a reduced life expectancy and an increased risk of developing complications.
The ideal diagnostic work-up starts with the clinical suspect of the disease. In classical AFD, the cardiac phenotype is usually associated with noncardiac manifestations such as renal dysfunction, TIA or cryptogenic stroke. Fabry facies is unusual and more common in male than in female patients; when present, it is characterized by prominent supraorbital ridges, bushy eyebrows, widened nasal bridge and bulbous nasal tip, recessed forehead, shallow midface, full lips, coarse features, prognathism and posteriorly rotated ears. Skin angiokeratomas are prevalently (but not exclusively) located in typical ‘bathing suit’ areas; labial and proximal nail fold telangiectasia can be present. Deep exploration of the clinical history usually highlights abdominal crises of pain starting in infancy, and heat intolerance, with acral painful episodes. In atypical or variant forms of AFD (cardiac, renal, nervous), the phenotype is characterized by prevalent involvement of one organ, which makes the clinical diagnostic hypothesis difficult to be formulated. However, deep phenotyping may demonstrate abnormalities of other organs/tissues. Multidisciplinary evaluation including ophthalmology, cardiology, neurology, nephrology, dermatology is usually activated when the first clinician that observes the patient suspects a systemic disease. Therefore, the clinical skill is a major contributor to clinically based diagnostic hypothesis.
The Fabry heart
Irrespective of symptoms, the cardiologic diagnostic work-up includes cardiology visit, baseline ECG, imaging [two dimensional-transthoracic echocardiography and cardiac magnetic resonance (CMR) when needed] and evaluation of possible arrhythmias. The heart is involved in up to 70% of patients. AFD cardiac manifestations often mimic HCM, for which no specific treatment exists. A disease-oriented diagnostic mindset is essential to clinically suspect AFD and highlight differences of AFD heart from sarcomeric HCM. The MOGE(s) (the morphofunctional phenotype (M), organ(s) involvement (O), genetic inheritance pattern (G), etiological annotation (E) including genetic defect or underlying disease/substrate, and the functional status (S) of the disease) cardiomyopathy classification system7 (that considers cardiac morpho-functional phenotype together with extracardiac organ involvement, familial inheritance pattern and etiological description) can guide the clinical evaluation and allows annotation of cardiac and noncardiac phenotypic traits.
Electrocardiography
A short PR interval may be one of the first signs of cardiac involvement and has been shown to be due in particular to shortening of P-wave duration.8 Short PR interval could be found in 20–40% of adult AFD patients and in about 30% of pediatric AFD patients. Atrio-ventricular and intraventricular intervals may increase with age and may become prolonged in advanced phases of the disease, more likely reflecting a progressively increasing disease burden and age-related degenerative process. Electrocardiographic parameters may change together with macroscopic myocardial changes (i.e. prolonging PR with left atrial enlargement due to LV hypertrophy with diastolic dysfunction) losing their diagnostic relevance in the differential diagnosis of the LV hypertrophy. The use of PR interval minus P-wave duration in lead II has been proposed as an index useful to overcome the impaired diagnostic value of PR duration in AFD patients with enlarged left atrial dimension. The specificity of this latter index remains to be confirmed in larger series. In adults, ECG signs of LV hypertrophy are present in up to 60% of men and 18% of women and can be associated with repolarization abnormalities. These changes are observed in lateral leads (V5 and V6) in patients with LV hypertrophy and late gadolinium enhancement (sign of focal fibrosis) in CMR as well as in patients without echocardiographic signs of LV hypertrophy and no evidence of focal scarring. Abnormal low voltages on ECG (i.e. the total sum of QRS amplitude in DI, DII, DIII < 1.5 mV) are not typical of AFD patients and this sign could be useful to consider the diagnosis unlikely.
Ambulatory ECG monitoring
The most common arrhythmia in patients with AFD is sinus bradycardia, followed by ectopic rhythm. Increasing age has been demonstrated to be associated with progressive sinus and atrio-ventricular node disease necessitating a close monitoring for bradyarrhythmias and the implantation of a pacemaker.11 Therefore, bradyarrhythmias are common in adult male patients, most of all in the late phase of the disease. Arrhythmias, including atrial fibrillation and ventricular tachycardia, occur in about 30–40% of AFD patients; of note, they can be encountered in patients with preserved LV function and in the absence of LV hypertrophy or valve disease.
Nonsustained ventricular tachycardia and nonspecific intraventricular conduction disturbances are detectable in almost all affected adults. The recurrence of arrhythmias is independent of the presence of LV hypertrophy. Sudden cardiac death related to ventricular arrhythmia is uncommonly observed but remains the most life-threatening condition even if a rather more important role for bradycardia has been proposed.
Echocardiography
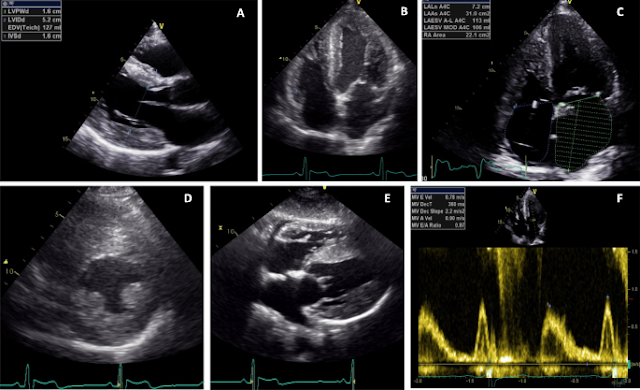
LV mild-to-moderate concentric hypertrophy (14–20 mm) is the most common finding in affected adult patients (Fig. 1). Among AFD patients with concentric LV hypertrophy, the prominence of papillary muscles and the ratio between papillary muscle size to LV circumference have been proposed as echocardiographic markers for diagnosing AFD. Their combination has yielded a sensitivity of 75% and a specificity of 86% for diagnosing AFD with LV hypertrophy.13 LV remodeling or hypertrophy has been demonstrated in male and female patients since adolescence. Anyway, even in classic AFD male patients (those with the most severe phenotype), the maximum diastolic interventricular septum thickness is unlikely to be over 15 mm below 20 years of age. For this reason, severe LV hypertrophy (>15 mm) below 20 years of age could be a feature that makes the diagnosis of AFD unlikely. Progressive systolic and diastolic dysfunction becomes evident with increasing age. Systolic and diastolic tissue Doppler imaging indexes show early reductions before the onset of myocardial hypertrophy; usually the longitudinal performance is impaired before the radial.
Johannes Fabry (1860-1930), German dermatologist.
 |
| Johannes Fabry |
Johannes Fabry studied medicine in Bern and Berlin. He received his doctorate in 1886, and after gaining his medical qualification, Fabry trained in dermatology at the Royal Clinic for Skin and Venereal Disease in Bonn under Joseph Doutrelepont (born 1834), and subsequently in Zürich with Hugo Ribbert (1855-1920). From 1889 to 1927 he was principal medical officer of the Skin Clinic at the Dortmund city hospital, which he developed into a leading centre for that specialty. A charismatic teacher, he attracted many postgraduate students. Fabry's work covered almost every field of dermatology.
William Anderson (1842-1900), English surgeon and dermatologist.
 |
| William Anderson |
William Anderson was educated at the City of London School. He then entered the University of Aberdeen, but soon moved to the Lambeth School of Art, where he developed his considerable talents as a draftsman and artist, and obtained a medal for artistic anatomy. He entered medical school at St. Thomas’ Hospital in 1864, graduating in 1867 with the Cheselden Medal for surgery. He was a hard-working student and, being somewhat reticent, preferred the wards and classrooms to boisterous extra-curricular activities.
He was conferred doctor of medicine in 1868 and then worked as a house surgeon at St. Thomas’ Hospital. He became a fellow of the Royal College of Surgeons in 1869 and thereafter gained practical surgical experience at the General Hospital, Derby, before returning to St. Thomas’ Hospital in 1871 as surgical registrar and demonstrator in anatomy. His appointment coincided with the opening of the new hospital, which was situated on the south bank of the river Thames opposite the Houses of Parliament. His artistic abilities proved to be of great value in the illustration of his lectures on anatomy and he quickly acquired a reputation for his prowess in this field. His students were especially impressed by his ability to draw on the blackboard simultaneously with both hands.
References
1: Di Toro A, Favalli V, Arbustini E. Anderson-Fabry disease. J Cardiovasc Med
(Hagerstown). 2018 Feb;19 Suppl 1:e1-e5. doi: 10.2459/JCM.0000000000000637.
PMID: 29538136.
2: Simonetta I, Tuttolomondo A, Di Chiara T, Miceli S, Vogiatzis D, Corpora F,
Pinto A. Genetics and Gene Therapy of Anderson-Fabry Disease. Curr Gene Ther.
2018;18(2):96-106. doi: 10.2174/1566523218666180404161315. PMID: 29618309.
3: Chan B, Adam DN. A Review of Fabry Disease. Skin Therapy Lett. 2018
Mar;23(2):4-6. PMID: 29562089.
4: Schiffmann R. Fabry disease. Handb Clin Neurol. 2015;132:231-48. doi:
10.1016/B978-0-444-62702-5.00017-2. PMID: 26564084.
5: El-Abassi R, Singhal D, England JD. Fabry's disease. J Neurol Sci. 2014 Sep
15;344(1-2):5-19. doi: 10.1016/j.jns.2014.06.029. Epub 2014 Jun 21. PMID:
25106696.
6: Perry R, Shah R, Saiedi M, Patil S, Ganesan A, Linhart A, Selvanayagam JB.
The Role of Cardiac Imaging in the Diagnosis and Management of Anderson-Fabry
Disease. JACC Cardiovasc Imaging. 2019 Jul;12(7 Pt 1):1230-1242. doi:
10.1016/j.jcmg.2018.11.039. Erratum in: JACC Cardiovasc Imaging. 2019
Sep;12(9):1903. PMID: 31272606.
7: Stephan F, Haber R. Maladie de Fabry [Fabry disease]. Ann Dermatol Venereol.
2017 Feb;144(2):137-146. French. doi: 10.1016/j.annder.2016.10.010. Epub 2017
Jan 16. PMID: 28104284.
8: Anderson-Fabry disease. Lancet. 1990 Jul 7;336(8706):24-5. PMID: 1973214.
9: Zarate YA, Hopkin RJ. Fabry's disease. Lancet. 2008 Oct 18;372(9647):1427-35.
doi: 10.1016/S0140-6736(08)61589-5. PMID: 18940466.
Tags: fabry anderson disease mayo clinic, fabry anderson disease icd 10, fabry-anderson disease current state of knowledge, anderson fabry disease radiology, anderson fabry disease symptoms, anderson fabry disease nhs, anderson fabry disease treatment, anderson-fabry disease cardiac mri, fabry anderson disease, anderson-fabry disease (afd), anderson-fabry disease a multiorgan disease, what is anderson fabry disease, fabry anderson syndrome, anderson fabry disease cmr, what is fabry anderson disease, anderson fabry disease diagnosis, how do you diagnose fabry disease, how to diagnose fabry disease, anderson-fabry disease extrarenal neurologic manifestations, anderson-fabry disease ecg, fabry disease manifestations, anderson-fabry disease in heart failure, how does fabry disease affect the heart, anderson fabry disease heart, anderson fabry disease mri, what is fabry disease nhs, fabry anderson syndrome symptoms, fabry disease x linked, type 2 fabry disease
No comments:
Post a Comment