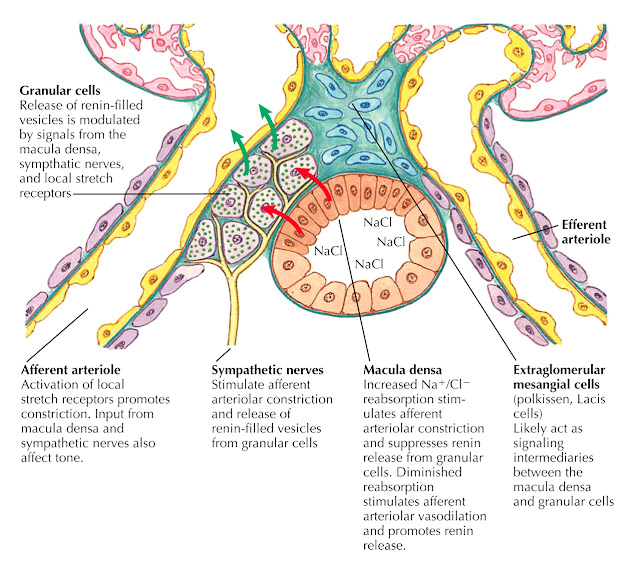
The available evidence suggests that the macula densa, located at the end of the thick ascending limb, senses tubular flow based on the concentrations of sodium and chloride in the local filtrate. The sensing apparatus appears to be apical Na+/K+/2Cl− (NKCC2) cotransporters.
 |
| Tubuloglomerular feedback and modulation of renin release |
When tubular fl ow rates are high, there is a slight decrease in solute reabsorption before the macula densa, and thus higher concentrations of sodium and chloride are present at this area. Increased activation of NKCC2 transporters ensues, which leads to constriction of the afferent arteriole and inhibition of renin release.
📖 Critical Care Nephrology 3rd Edition
In contrast, when tubular fl ow rates are low, decreased activation of NKCC2 transporters leads to dilation of the afferent arteriole and activation of renin release. The exact signals that connect the NKCC2 transporters of the macula densa to the afferent and efferent arterioles remain poorly understood; however, there is increasing evidence that adenosine plays a key role. In one proposed model, increased reabsorption by NKCC2 transporters stimulates basolateral Na+/K+ ATPases.
The increased ATP consumption yields ADP and AMP, which local proteins convert into adenosine. Adenosine, in turn, activates receptors on the surface of nearby extraglomerular mesangial cells, causing an increase in intracellular calcium levels. A wave of intracellular calcium is transmitted across gap junctions to the smooth muscle and granular cells of the afferent and efferent arterioles, causing constriction of the afferent arteriole and inhibition of renin release.
In contrast, when there is low tubular fl ow and diminished reabsorption by NKCC2 transporters, the adenosine signal is eliminated, leading to dilation of the afferent arteriole and stimulation of renin release. In addition, there is some evidence that macula densa cells contain COX-2 enzymes that are also stimulated when there is diminished reabsorption by NKCC2 transporters; these appear to synthesize prostaglandins that stimulate dilation of the afferent arteriole and promote renin release.
References
- 1 Bell PD, Lapointe JY, and Peti-Peterdi J. Macula densa cell signaling. Annu Rev Physiol 65: 481–500, 2003.
Crossref | PubMed | ISI | Google Scholar - 2 Bell PD, Lapointe JY, Sabirov R, Hayashi S, Peti-Peterdi J, Manabe K, Kovacs G, and Okada Y. Macula densa signaling involves ATP release through a maxi anion channel. Proc Natl Acad Sci USA 100: 4322–4327, 2003.
Crossref | PubMed | ISI | Google Scholar - 3 Brown R, Ollerstam A, Johansson B, Skott O, Gebre-Medhin S, Fredholm B, and Persson AE. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol 281: R1362–R1367, 2001.
Link | ISI | Google Scholar - 4 Miller JA. Renal response to sodium restriction in patients with early diabetes mellitus. J Am Soc Nephrol 8: 749–755, 1997.
PubMed | ISI | Google Scholar - 5 Nishiyama A and Navar LG. ATP mediates tubuloglomerular feedback. Am J Physiol Regul Integr Comp Physiol 283: R273–R275, 2002.
Link | ISI | Google Scholar - 6 Olivera A, Lamas S, Rodriguez-Puyol D, and Lopez-Novoa JM. Adenosine induces mesangial cell contraction by an A1-type receptor. Kidney Int 35: 1300–1305, 1989.
Crossref | PubMed | ISI | Google Scholar - 7 Osswald H, Nabakowski G, and Hermes H. Adenosine as a possible mediator of metabolic control of glomerular filtration rate. Int J Biochem 12: 263–267, 1980.
Crossref | PubMed | Google Scholar - 8 Osswald H, Spielman WS, and Knox FG. Mechanism of adenosine-mediated decreases in glomerular filtration rate in dogs. Circ Res 43: 465–469, 1978.
Crossref | PubMed | ISI | Google Scholar - 9 Riser BL, Cortes P, Zhao X, Bernstein J, Dumler F, and Narins RG. Intraglomerular pressure and mesangial stretching stimulate extracellular matrix formation in the rat. J Clin Invest 90: 1932–1943, 1992.
Crossref | PubMed | ISI | Google Scholar - 10 Schnermann J and Briggs JP. Function of the juxtaglomerular apparatus: control of glomerular hemodynamics and renin secretion. In: The Kidney: Physiology and Pathophysiology (3rd ed.), edited by Seldin DW and Giebisch G. Philadelphia: Lippincott Williams & Wilkins, 2000, p. 945–980.
Google Scholar - 11 Schnermann J and Levine DZ. Paracrine factors in tubuloglomerular feedback: adenosine, ATP, and nitric oxide. Annu Rev Physiol 65: 501–529, 2003.
Crossref | PubMed | ISI | Google Scholar - 12 Schnermann J, Weihprecht H, and Briggs JP. Inhibition of tubuloglomerular feedback during adenosine1 receptor blockade. Am J Physiol Renal Physiol 258: F553–F561, 1990.
Link | ISI | Google Scholar - 13 Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, and Schnermann J. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci USA 98: 9983–9988, 2001.
Crossref | PubMed | ISI | Google Scholar - 14 Thomson S, Bao D, Deng A, and Vallon V. Adenosine formed by 5′-nucleotidase mediates tubuloglomerular feedback. J Clin Invest 106: 289–298, 2000.
Crossref | PubMed | ISI | Google Scholar - 15 Thomson SC, Deng A, Bao D, Satriano J, Blantz RC, and Vallon V. Ornithine decarboxylase, kidney size, and the tubular hypothesis of glomerular hyperfiltration in experimental diabetes. J Clin Invest 107: 217–224, 2001.
Crossref | PubMed | ISI | Google Scholar - 16 Vallon V. Tubuloglomerular feedback in the kidney: insights from gene-targeted mice. Pflügers Arch 445: 470–476, 2003.
Crossref | PubMed | ISI | Google Scholar - 17 Vallon V, Blantz RC, and Thomson S. Homeostatic efficiency of tubuloglomerular feedback is reduced in established diabetes mellitus in rats. Am J Physiol Renal Physiol 269: F876–F883, 1995.
Link | ISI | Google Scholar - 18 Vallon V, Blantz RC, and Thomson SC. Glomerular hyperfiltration and the salt paradox in early diabetes mellitus: a tubulo-centric view. J Am Soc Nephrol 14: 530–537, 2003.
Crossref | PubMed | ISI | Google Scholar - 19 Vallon V, Huang DY, Deng A, Richter K, Blantz RC, and Thomson SC. Salt-sensitivity of proximal reabsorption alters macula densa salt and explains the paradoxical effect of dietary salt on glomerular filtration rate in diabetes mellitus. J Am Soc Nephrol 13: 1865–1871, 2002.
Crossref | PubMed | ISI | Google Scholar - 20 Vallon V, Richter K, Blantz RC, Thomson S, and Osswald H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol 10: 2569–2576, 1999.
- Tags: tubuloglomerular feedback mechanism, tubuloglomerular feedback vs glomerulotubular balance, tubuloglomerular feedback quizlet, tubuloglomerular feedback definition, tubuloglomerular feedback mechanism quizlet, tubuloglomerular feedback from the dct is when, tubuloglomerular feedback function, tubuloglomerular feedback explained, tubuloglomerular feedback and renin, tubuloglomerular feedback adenosine, tubuloglomerular feedback and myogenic response, tubuloglomerular feedback and glomerulotubular balance, tubuloglomerular feedback and juxtaglomerular apparatus, tubuloglomerular feedback and balance, tubuloglomerular feedback arterial blood pressure, tubuloglomerular feedback also known as, the tubuloglomerular feedback mechanism, what is tubuloglomerular feedback, what is a feedback mechanism, what are the three components of a feedback mechanism
No comments:
Post a Comment